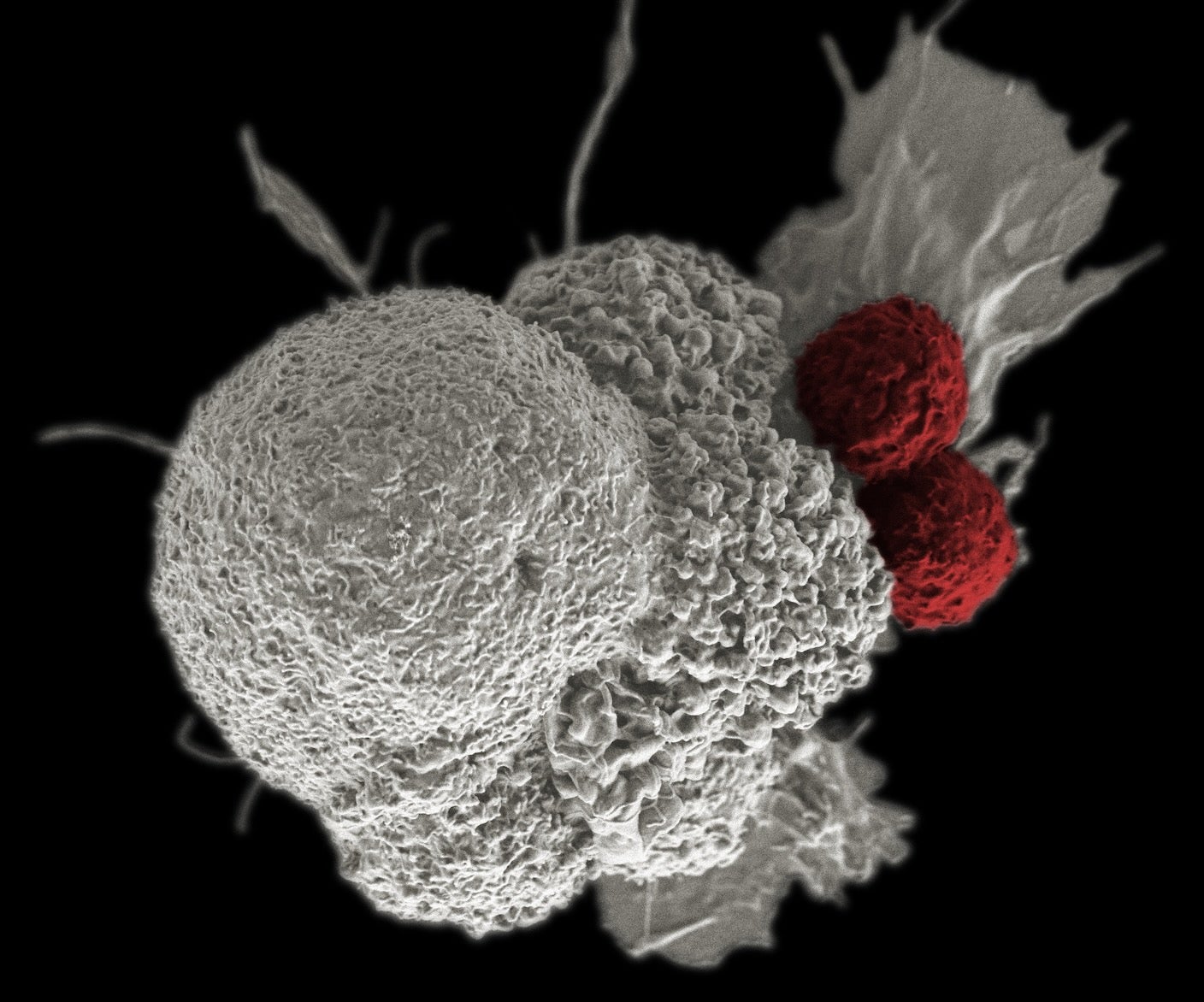
Cancer cells steal from and poison the cells tasked with fighting them off.
Cancers are sneaky infiltrators highly adept at cellular warfare. As they expand, the malignant cells chip away at the body’s immune defenses.
How cancer cells learn to dodge immune attacks has puzzled scientists for decades. A study in Nature this week has a surprising answer: They steal healthy mitochondria—the cell’s energy powerhouses—from the immune cells that hunt them down. In turn, cancers pump their own damaged mitochondria into healthy immune cells, gradually destroying them from the inside.
Scientists have always assumed mitochondria are produced inside cells and live out their lives there. The new findings challenge this dogma, suggesting mitochondria are mobile—at least in cancers and the tumor-infiltrating immune cells working to fight them off.
Analyzing both types of cells from three cancer patients, the Japanese team found that cancer-fighting immune cells poisoned with damaged mitochondria eventually lose their ability to resist. Without a healthy energy source, the cells languish in a state called senescence, where they can no longer function or divide.
Meanwhile, cancer cells pilfer healthy mitochondria from their attackers to satisfy their appetite for energy, allowing them to divide and spread. In tests in petri dishes and mice, the team found that blocking this mitochondria swap slowed cancer growth and made a common immunotherapy more effective.
Stanford’s Holden Maecker, who was not involved in the study, told Nature the finding “sounds crazy, like science fiction,” and that it’s “potentially a totally new biology that we were not looking at.”
This swapping “is a newly discovered mechanism that thwarts anticancer defenses,” wrote Jonathan Brestoff at Washington University School of Medicine, who was also not part of the study.
Energy Mill
Mitochondria are a crucial ingredient of life. These oblong-shaped organelles are loaded with proteins that convert fuel from food into energy. Unlike other organelles, mitochondria stand out because they carry their own genetic material.
Let’s backtrack, for a moment.
Most of our DNA is housed in the cell’s nucleus. But mitochondria also contain their own genes, dubbed mtDNA. These are likely remnants of their past. A common theory is that mitochondria were originally independent cells that were “eaten up” by another early type of cell at the beginning of complex life on Earth. Eventually the two formed an alliance, with mitochondria increasing energy production for the cells, and the cells protecting the mitochondria.
Today, mtDNA mostly operates independently from our cells’ nuclear genetic material. It’s stored in circles of DNA (like in bacteria). Unlike the rest of our DNA, mtDNA never evolved sophisticated repair mechanisms, and it’s prone to accumulating mutations.
As they produce energy, mitochondria also damage themselves by pumping toxic waste into their surroundings. Cells routinely dispose of defunct mitochondria to make space for healthy replacements that can keep the cellular factory—and our bodies—humming along.
This process goes awry in cancer.
Cops and Robbers
Cancer cells grow incessantly. They require a steady stream of energy to keep up with their need to repeatedly copy their DNA to divide, grow, and spread. But mitochondria in cancer cells are often mutated and struggle to supply their demanding hosts with enough energy.
In recent decades, scientists have noticed that some cells can shuttle their mitochondria to others. Mostly, the process seems to help out a struggling neighbor. But early hints also suggested mitochondrial transfers could contribute to cancer growth. One study found that tumor cells connect to healthy immune cells to siphon off mitochondria, depleting their attackers of energy while bolstering their own—at least in petri dishes.
Whether this happens in cancer patients is controversial. The new study paints a clearer picture.
The team took samples of tumors and cancer-fighting immune cells—tumor-infiltrating lymphocytes (TILs), in this case—from three cancer patients and analyzed their mtDNA makeup. Normally, each type of cell harbors its own mtDNA mutational “barcode.”
For each patient, both cell types shared the same cancerous barcode—suggesting that mitochondria from the tumors might be hopping to, and taking over, their attackers.
As the team watched the cells—now growing together in the lab—they found cancer mtDNA almost completely replaced native DNA in some of the immune cells. The team also found the cancer cells were stealing healthy mitochondria from their immune attackers by sending out nanotubes that burrowed into them. Meanwhile, the cancer cells spewed their own damaged mitochondria, encapsulated in fatty bubbles, towards the immune cells.
“These findings establish the first clear evidence of bidirectional exchange of mitochondria between two cell types,” wrote Brestoff.
Damaged mitochondria don’t often linger inside healthy cells. They’re rapidly shuttled to the cellular “trash bin.” Those inherited from cancer cells, however, were dotted with a protein that hid them from the cells. Like leaking chemical plants, the mutated mitochondria silently festered inside without detection.
Over time, the hijacked immune cells slowly degraded. No longer able to divide, they entered “senescence”—a zombie-like state where they excreted a toxic protein soup that further lowered their cancer-battling abilities. In short, by robbing these cells of healthy mitochondria, cancer cells turned the body’s first-line defense into an ally that helped them grow.
Cutting the Line
The team next dotted tumor mitochondria in tumors with a glow-in-the-dark protein to track them and implanted them into mice.
They found that immune cells in the mice containing cancer-derived mitochondria were far less effective at fighting off cancer cells. They were “exhausted,” explained the team. The contaminated cells struggled to maintain enough energy to ward off cancers and could no longer replicate. However, drugs that blocked mitochondrial transfer revitalized the exhausted cells and made treatment with a common cancer immunotherapy more effective.
Though the results are in mice, mitochondrial transfer could also play a previously unrecognized role in human cancers. Analyzing clinical data from roughly 200 people with two types of cancer, the team found that increased mtDNA mutation was associated with worse outcomes, even when the patients were receiving immunotherapy treatments.
Mitochondrial health has often been studied in the context of aging. These results could spur new interest in how it impacts cancer and other diseases. We still don’t know how exactly mitochondria from cancers damage immune cells. But further study could potentially inspire new treatments to block mitochondrial swaps. Myriad tools already exist to track mitochondria. Expanding research into cancer biology would be a relatively easy next step.
Although “it remains to be determined how prevalent such mitochondrial exchange is” between other cell types, Brestoff wrote, the research raises new questions about its role in other diseases.
The post Scientists Say They’ve Discovered How Cancer Hijacks and Corrupts Immune Cells appeared first on SingularityHub.